×
Abbreviations
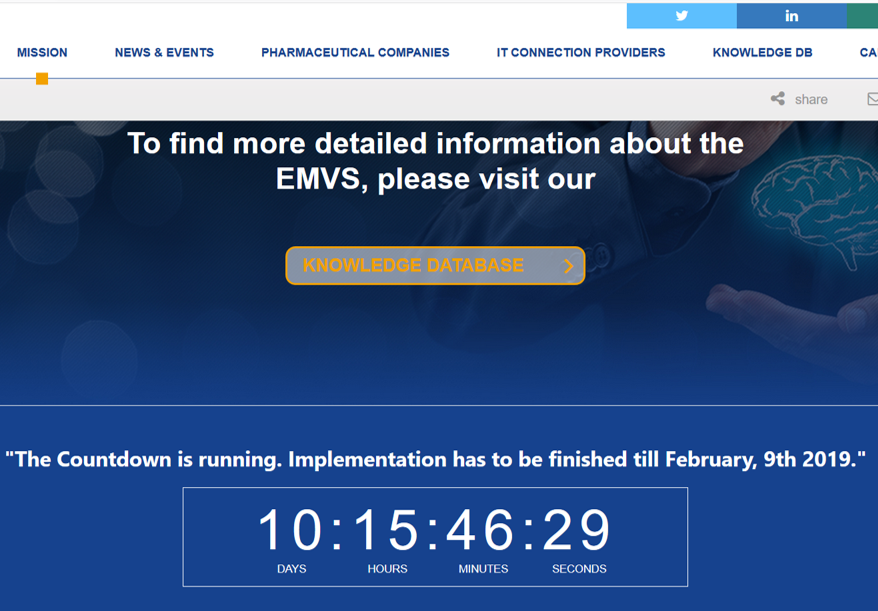
FMD – Falcified medicines directive –
Directive 2011/62/EU of the European Parliament and of the Council of 8 June 2011 amending Directive 2001/83/EC on the Community code relating to medicinal products for human use, as regards the prevention of the entry into the legal supply chain of falsified medicinal products
EMVS – European Medicines
Verification System (consists of a central part that is interfaced with various national fatabases)
NMVS – National Medicines Verification System – (interfaced with the EMVS)
Arvato – development partner of REKS (developer of the Blueprint solution)
BP – Blue Print – technical solution of the system
EtMVS – Estonian Medicines Verification System
REKS – Estonian Medicines Verification Organisation
RTL – Association of Pharmaceutical Manufacturers of Estonia
Efpia – European Federation of Phamaceutical Industries and Associations
MfA – Medicines for Europe
GIRP - Groupement International de la Repartition Pharmaceutique
EAEPC - European Association of Euro-Pharmaceutical Companies
PGEU - Pharmaceutical Group of the European Union
HOPE - European Hospital and Healthcare Federation